Most tryptic peptides have molecular weights (MW) below 2 kDa. Both ion traps from Thermo and Bruker (LTQ from Thermo for peptides with MW<2 kDa and Bruker’Amazon ion trap- for peptides with MW<3.5 kDa) can be used to reveal peptide amino acid sequence using MS and MS/MS modes. A statistical approach can be used to evaluate quality of MALDI mass spectra (spot-to-spot signal repeatability for same samples, etc) obtained using a particular sample preparation method 1
Limit of detections (LODs) for tryptic peptides in AP MALDI MS method
LODs of peptides prepared using CHCA and analyzed in Atmospheric Pressure (AP) MALDI source hyphenated with LTQ (Thermo) MS platform are typically between 1 and 10 femtomole of peptides loaded to 1 uL of CHCA solution (i.e., the minimum “detectable” peptide concentration is between 10-8 M to 10-9 M).
When using CHCA as MALDI matrix, phosphopeptides (e.g., tryptic phosphopeptides) have LODs approximately 5-10 fold lower than their non-phosphorylated analogs. When analyzing phosphopeptides, it is recommended to add phosphoric acid to matrix solution2 (up to1%) or 1 mM of NH4H2PO43.
Chlorinated CHCA (modified CHCA) can be used to improve the MS analysis of some peptides4,5.
Improving peptide LODs by using lower matrix concentrations in dried droplet samples
LODs proportionally improve when matrix solution becomes less concentrated, that is, the matrix solution is prepared with lower matrix content (e.g., 0.1-1 mg/mL)6. Yet with very low matrix concentration, one ends up with just a few micro-crystals over the dried droplet area (\~ 1×1 mm2). The crystals can be easily consumed after a few dozen of laser shots. In case of 1mg/mL or lower concentrations of CHCA in a combined solution, the distribution of sample microcrystals over a dried droplet spot does not look as a field densely populated with matrix microcrystals, but rather as a group of similar but scattered crystals with the 10-50 micron voids between individual micro-crystals. So when scanning such a sample spot with \~20 micron diameter laser beam, one can see very large variations in MS signal due to the fact that laser beam can hit or miss individual crystals. The increase in signal-to-noise in mass spectra with lower matrix content in a droplet has been attributed to using more favorable analyte-to- matrix ratios7 (e.g., moving from 1:10,000 to 1:1000).
When using low-concentration matrix solutions, the increase in LODs with better signal statistics (more smooth signals) can be achieved through a use of special target plates that have small circular hydrophobic patches8 (0.3-0.8 mm in diameter) encircled by a hydrophilic ring (e.g., Anchor Chips9). In this case, 1-10 uL droplets are pipetted onto circular hydrophobic patches where the droplets slowly dry eventually producing a dense field of micro-crystals (see for instance Bruker’s guide to MALDI sample preparation 10).
The lack of homogeneity problem in MALDI samples
The dried-droplet method is the most popular method to prepare MALDI samples because it is rather simple and quick. In case of CHCA or alkylated CHCA (alkylated DHB), it often produces good enough results for different types of peptide samples. In many cases, however, the formed crystals of other “popular” matrices (notoriously known for its large crystals is DHB) are large and are characterized by very inhomogeneous size distribution. Moreover, these crystals are unevenly distributed over the dried droplet spot, so ion signal intensities from such spots widely vary depending on a chance that the laser beam illuminates the “right” kind of crystals (so-called “sweet spots”, or matrix crystals heavily doped with peptides )11,12. In this regard, the dried droplet method applied to matrices other than CHCA has a limited usage for accurate quantitative mass analysis applications13.
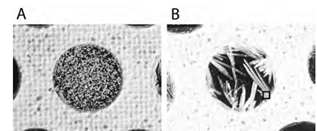
Crystals of CHCA (A) and DHB (B). The marked square in panel B indicates the “sweet” spot producing signals with high S/N ratio in MALDI MS analysis
Improving LODs using other sample preparation methods
Reduction in crystal size by using E-wetting process
Improvements to making more homogenous dried droplet samples can be done by using forced steering or by adding nucleation-inducing microparticles such as soot. The first is done by applying smooth strokes with micro-pipette14 while the latter used microparticles of carbon (soot)15. Alkylated matrices16,17 can also provide smaller crystals, yet these matrices are more hydrophobic compared to their “normal” analogs, so these alkylated matrixes are more efficient for the analysis of large or hydrophobic peptides and small proteins.
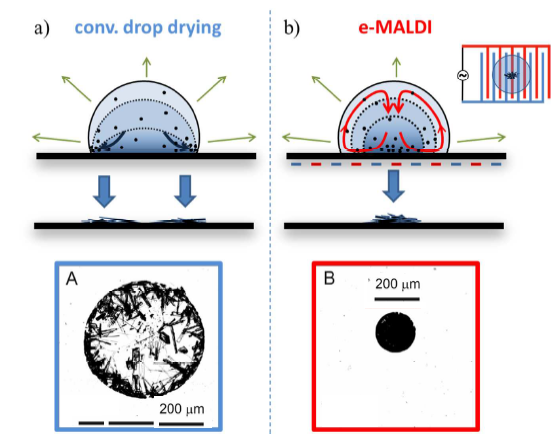
More compact and more homogeneous sample spots can be prepared by using electro-wetting technique, where the surface of non-conductive MALDI plate has deposited interlaced narrow metal electrodes. The application of AC voltage between the adjacent pairs of narrow metal electrodes causes the liquid micro-circulation in the liquid droplet thus preventing the crystals to grow to a large size 18.
Reduction in crystal size by using sublimation process
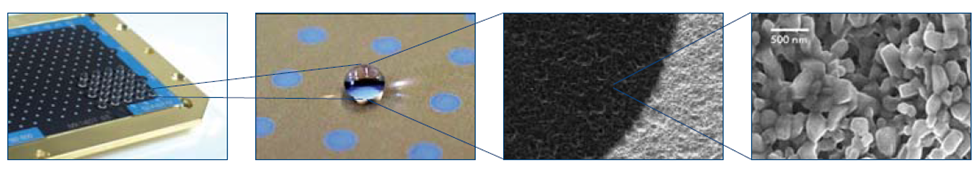
Separately should be mentioned sample preparation methods that utilize matrix sublimation on a hydrophobic surface. Typically the hydrophobic spots are produced by vaporizing a thin layer of gold19 on microscopic slides to make glass surface both hydrophobic and conductive.
The thin matrix layer (the “base” layer) can be prepared also by using tetrahydrofuran concentrated (20 mg/mL) CHCA solution20 spotted on a golden surface. Peptides are dissolved in water (0.1% TFA) solution containing only 1 % of acetonitrile. Aliquots (1 uL) of peptide solution (water-only solutions with 0.1% TFA, with maximum 1 % of Acetonitrile) are placed on the top of sublimated CHCA layer.
The main advantage of delivering small (1-3 uL) aliquots of peptide solutions onto 0.7-mm diameter spots obtained by vacuum sublimation of matrix is 10-fold (at least, some reports mentioned even 50-fold increase) higher analyte sensitivities21, 22 compared to standard dried droplet preparation method. This is likely related to the formation of considerably smaller crystals (with average size\< 1 um), which characterized by a very narrow crystal size distribution as compared to dried droplet preparation which typically gives much larger size of crystals with broader size distribution. In addition, using mostly water solution of peptides (adding maximum of 1 percent of Acetonitirle) dropped on a top of CHCA layer provides conditions of re-dissolving only the matrix crystals in the upper layer. So after water evaporates, a new layer of small crystals forms on the top on old ones. In these crystals, the analyte-to-matrix ratio becomes very high which leads to high ion signals with fewer matrix peaks. The technique of vacuum deposition of CHCA followed by deposition of water-based peptide solution on the top of vaporized matrix layer was originally developed by a research group from Germany23. In 2006, it was licensed to Qiagen. At the moment, the plates similar to Qiagen ones are commercially available from Hudson Surface Technologies (muFocus plates, www.maldiplate.com). Those plates have pre-loaded CHCA or DHB spots from 700 to 1000 micron in diameter.
Using special materials or coatings in MALDI target plates to improve signal
Using the pyrographite sheets (PGS) or graphene sheets placed on a surface of stainless steel target was shown to improve the improvement in dried droplet sample morphology, especially with DHB samples. The resulting morphology of the dried droplet is the field of extremely small and uniform crystals. This leads to better reproducibility and quantification of MALDI samples24.
Desalting MALDI samples
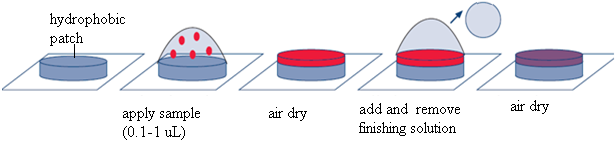
Salt reduction step. To reduce the salt content in sample spots and thus to reduce “chemical noise” in MALDI spectra, the dip is recommended of the plate with MALDI samples into cold (4 C) “finishing” solution or place a droplet of cold finishing solution onto the sample spot, wait for 3-5 sec, then remove the liquid with a pipette tip. Finishing solution is typically water containing 20mM of NH4H2PO4.
Mixture of Matrices
Hydrophobic peptides, such as those comprising parts of proteins traversing cell membranes, are generally difficult to detect using matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) because the majority of MALDI matrixes are hydrophilic or mildly hydrophobic and therefore have a low propensity to co-crystallize with hydrophobic peptides. There is a matrix additive, O-alkylated dihydroxybenzoic acid (ADHB), which is a 2,5-dihydroxybenzoic acid (DHB) derivative incorporating a hydrophobic alkyl chain on a hydroxyl group, that improves detection of hydrophobic peptides, yet only when mixed with CHCA, thereby improving MALDI-MS sensitivity25. The addition of ADHB to CHCA improved the sensitivity of hydrophobic peptides 10- to 100-fold. The sequence coverage of hydrophobic sections of proteins could be considerably increased using ADHB plus CHCA.
MS imaging indicated that hydrophobic peptides were incorporated into matrix crystals produced on the rim of a matrix/analyte dried droplet spot (on a stainless steel target plate) when ADHB +CHCA was used. Hydrophilic peptides, on contrary, were spread overall the dried droplet spot
In this experiment, CHCA was dissolved in 50/50 AcN/0.1% aqueous TFA (v/v) at 10 mg/mL. Alcylated DHB (aDHB) modified with a C8 alkyl chain via O atom of one of two DHB’s OH groups was synthesized and prepared in 50/50 (v/v) ACN/0.1% aqueous TFA at 5 mg/mL. 10 parts of CHCA solution were mixed with one part of aDHB. Peptide solution (50/50 (v/v) ACN/0.1% aqueous TFA) was added to the “combined” matrix solution at ratio 1:10. LOD for hydrophobic peptides was reported to be 0.1 fmole loaded into 1 uL droplet.
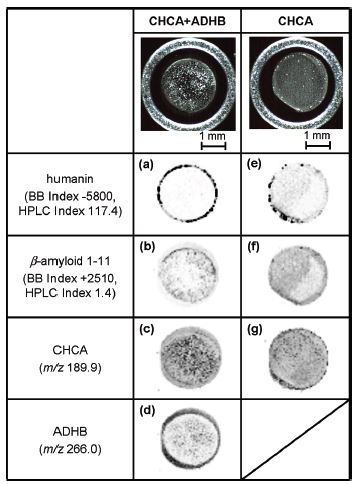
Matrix/analyte images (top rows) of dried droplets on a stainless steel target plate. Ion images of:* (a, e) hydrophobic peptide humanin,
- (b, f) hydrophilic peptide β-amyloid 1-11
- (c, g) CHCA,
- (d) aDHB in sample of a 10 fmol equimolar mixture of humanin and β-amyloid using CHCA + ADHB or CHCA alone
General sample preparation recommendations
Despite many advantages of MALDI method often referred as a quick method of mass analysis of dirty samples (the samples that are not purified by LC separation), obtaining a reasonably good result using this analytical technique depends largely on an appropriate choice of sample preparation method. MALDI MS analysis of complex biological samples such as cell lyophilisates requires careful optimization of the experimental conditions. The quality of mass spectra and thus the outcome of the analysis can be affected by the type and the amount of a MALDI matrix. The exact mass identification of a certain compound present in the sample should is typically based on a presence of signal with relatively high signal to noise peak in the spectrum. If enough material is available (e.g. the spot is not consumed during the MS analysis), the peptide under investigation can be subjected to CID or high energy CID to get data on a peptides sequence. The variety of peptides that can give such high intensity peaks is closely correlated with the matrix/sample ratio that was used in the sample preparation process. This can be explained by the fact that in a peptide mixture some peptides have better incorporation rate to matrix crystals than others. This incorporation will determine the local “neighborhood” of peptide molecule. Some analytes that present in abundance in a solution under analysis can be found to be co-crystallized with other analytes in the same matrix crystal leading to ion suppression. However, there is a range of matrix-to-sample ratios using which one can observe maximum number of high quality peptides peaks in MALDI mass spectra. Regarding to a limit of detection, the optimal matrix-to-sample ratio is different for particular peptides in the sample and should be determined individually.
- Kooijman PC, Kok SJ, Weusten J, Honing M. Independent assessment of matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) sample preparation quality: A novel statistical approach for quality scoring Anal Chim Acta, 2016; 919: 1-10 ↩
- Park S et al. Effect of phosphoric acid as a matrix additive in matrix-assisted laser desorption/ionization analysis. Rap Com Mass Spectrom. 2013;27(7):842-6. ↩
- Sample Preparation in Biological Mass Spectrometry, Eds: A. Ivanov and A. Lazarev. ↩
- Jaskolla TW, Papasotiriou DG, Karas M. Comparison between the matrices alpha-cyano-4-hydroxycinnamic acid and 4-chloro-alpha-cyanocinnamic acid for trypsin, chymotrypsin, and pepsin digestions by MALDI-TOF mass spectrometry, J Proteome Res 2009; 8(7):3588-97 ↩
- Fukuyama Y1, Nakajima C, Furuichi K, Taniguchi K, Kawabata S, Izumi S, Tanaka K. Alkylated trihydroxyacetophenone as a MALDI matrix for hydrophobic peptides. Anal Chem. 2013; 85(20):9444-8. ↩
- Hioki Y, Kuyama H, Hamana C, Takeyama K, Tanaka K.J Mass Spectrom. An improved sample preparation method for the sensitive detection of peptides by MALDI-MS 2013;48(11):1217-23. ↩
- Smolira A., Wessely-Szponder J. Importance of the Matrix and the Matrix/Sample Ratio in MALDI-TOF-MS Analysis of Cathelicidins Obtained from Porcine Neutrophils, Appl Biochem Biotechnol. 2015; 175: 2050–2065. ↩
- Owen SJ, Meier FS, Brombacher S, Volmer DA. Increasing sensitivity and decreasing spot size using an inexpensive, removable hydrophobic coating for matrix-assisted laser desorption/ionisation plates. Rapid Communications in Mass Spectrometry. 2003;17:2439-2449. ↩
- König S. Target coatings and desorption surfaces in biomolecular MALDI-MS. Proteomics 2008; 8(4):706-14. ↩
- Web site: https://researchservices.pitt.edu/sites/default/files/Bruker_Guide%20for%20MALDI_Sample_Preparation.pdf ↩
- Dai Y, Whittal R.M., Li L. Confocal Fluorescence Microscopic Imaging for Investigating the Analyte Distribution in MALDI Matrices, Anal Chem 1996; 68:2494-2500. ↩
- Garden R.W., Sweedler J.V., Heterogeneity within MALDI Samples As Revealed by Mass Spectrometric Imaging, Anal Chem. 2000; 72:30-36. ↩
- Duncan M.W., Roder H., Hunsucker S.W., Quantitative matrix-assisted laser desorption/ionization mass spectrometry, Brief Funct Genomic Proteomic, 2008; 7:355-370. ↩
- Avinash A. Patil, Cheng-Kang Chiang, Chen-Hao Wen, Wen-Ping Peng, Forced dried droplet method for MALDI sample preparation, Analytica Chimica Acta, 2018; 1031:128-133 ↩
- Gorka J, Bahr U, Karas M. Graphite supported preparation (GSP) of α-cyano-4-hydroxycinnamic acid (CHCA) for matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) for peptides and proteins. J Am Soc Mass Spectrom. 2012; 23(11):1949-54. ↩
- Fukuyama Y., Tanimura R., Maeda K., Watanabe M., Kawabata S., Iwamoto S., Izumi S., Tanaka K., Alkylated dihydroxybenzoic acid as a MALDI matrix additive for hydrophobic peptide analysis Anal Chem. 2012; 84(9):4237-43 ↩
- Wang S, Xiao Z, Xiao C, Wang H, Wang B, Li Y, Chen X, Guo X . (E)-Propyl α-Cyano-4-Hydroxyl Cinnamylate: A High Sensitive and Salt Tolerant Matrix for Intact Protein Profiling by MALDI Mass Spectrometry. J Am Soc Mass Spectrom. 2016; 27(4):709-18. ↩
- Kudina E, Burak-Eral H, Mugele F. E-MALDI: an electro-wetting-enhanced drop drying method for MALDI mass spectrometry, Anal. Chem., 2016; 88 (9): 4669–4675 ↩
- Poetsch A., Schlüsener D., Florizone C., Eltis L., Menzel C., Rögner M., Steinert K., Roth U. Improved Identification of Membrane Proteins by MALDI-TOF MS/MS Using Vacuum Sublimated Matrix Spots on an Ultraphobic Chip Surface. J Biomol Tech 2008; 19:129-138 ↩
- Lou X., de Waal B.F.M, Milroy L.G., van Dongen J. L. J. A sample preparation method for recovering suppressed analyte ions in MALDI TOF MS, J. Mass Spectrom. 2015, 50, 766–770 ↩
- Röhl H., Goethel S., Reihs K. MPep MALDI Chips for High-Sensitivity and High-Throughput Peptide Analysis by MALDI-TOF MS, Nature Methods, 2005; 2:6 ↩
- Jaskolla T., Roth U., Röhl H., Glückmann M., Karas M., Steinert K., Reihs K. Sensitive and Reproducible Detection of Peptides by MALDI-TOF MS Using Self-Assembled Monolayer (SAM)-Based Chips—Specifications and Applications*. Proceedings of the 54th ASMS Conference on Mass Spectrometry and Allied Topics* (May, 2006) ↩
- Jaskolla W, Karas M, Roth U, Steinert K, Menzel C, Reihs K. Comparison Between Vacuum Sublimed Matrices and Conventional Dried Droplet Preparation in MALDI-TOF Mass Spectrometry Journal of the American Society for Mass Spectrometry 2009; 20(6): 1104-1114 ↩
- Choi YK, Oh JY, Han SY. Large-Area Graphene Films as Target Surfaces for Highly Reproducible Matrix-Assisted Laser Desorption Ionization Suitable for Quantitative Mass Spectrometry. J Am Soc Mass Spectrom. 2018, Jul 11. ↩
